Light is fundamental to human existence. It delivers the Sun's energy to us without which life on Earth will be impossible. We use light to explore and understand our environment - vision is the only sense that comprehensively connects us to the outside world and is our main way of interacting with our surroundings. We rely on light to guide our behaviour and perceive the external world. The human brain uses 30-50% of its resources in processing vision related information
For 2000 years or more, wise men struggled to explain what light is. Historical attempts were interesting but generally wide off the mark - I refer you to 1, 2, 3, 4 for details. It is only in the past 400 years that good empirical evidence about light has been obtained. Detailed understanding of the nature of light has only been possible due to the pioneering work of many brilliant scientists like Newton, Maxwell, Einstein and others.
Dual Nature of Light - Funnily, sometimes light behaves like a beam of particles while at other times it behaves like a wave - this does not fit in the way we are used to making sense of objects in the world. Objects are either particles or spread out waves - they are never both. The enigmatic behaviour of light had caused untold confusion among scientists who were at a loss to understand if light is a stream of minute particles or is a wave travelling through the space between the emitter and the receiver. Within the framework of complex mathematical theories, we now can rationalise the dual nature of light - in fact, not only light but all matter (electrons, protons, neutrons, helium etc.) express this duality that has given us capabilities like electron microscopes etc.
Light is Energy - Light is transmission of energy. A source of light emits energy that travels to the receiver. The light energy may be transmitted as a wave (all waves carry energy) or as a stream of particles (called photons). Photons are little bundles of energy - the energy of a photon is determined by the frequency or wavelength of light (discussed in more detail later). Light energy interacts with electrons in the atoms and molecules of the receiver. Many possible things may happen in the interaction - for example, light may be absorbed or reflected or it may cause a chemical change etc. We shall look at some of these later.
Speed of Light - How fast light travels is a question that is now properly settled. If you open your eyes then you can see distant stars instantly - only possible if light travelled at an infinite speed, a view that most people held in the past. Clever experiments by Romer, Foucault and Fizeau determined that light travels with a finite speed - the modern agreed value of the speed of light (c) in vacuum is 299,792.458 km/sec. It is quick - covering the distance from the Sun to the Earth (~150 million km) in a mere 8 minutes and 20 seconds. Not only that - Einstein claims that this is the fastest that any object can possibly travel. Speed of light in vacuum (empty space) is the maximum speed possible. Prove Einstein wrong and you would cause a collapse of the modern science base!
By the way, when you look at a star, you are receiving light from it that left the star in the distant past - the light you receive now could have been travelling for a million years or ten billion years, depending on the distance of the star from the earth.
Colours - Light from the Sun appears white but pass it through a glass prism or a rain drop and one obtains a spectrum of colours (dispersion of light). Most credit for explaining the nature of colours goes to Newton and Maxwell.
The speed of light (v) in a transparent medium like glass or water is less than its speed in vacuum (c) . The relative change in speed defines a useful property of the medium - the refractive index (n = c/v).
When light travels from one medium to another, it encounters a change in refrative index - this causes a change in the direction of travel (bending of light or refraction). The speed and hence the bending depends on the wavelength of light - resulting in a separation of colours (dispersion). The slide explains how a spectrum is formed.
Dispersion of white light from the Sun by water drops in the atmosphere is responsible for the formation of one of the most beautiful phenomenon in nature - the rainbow. You can read all you need to know about rainbows in my blog by clicking here.
Theories of Light - While Newton (1643-1727) did most to measure the properties of light experimentally - his book Optiks published in 1704 provides details of his original and beautifully executed measurements, it was Maxwell (1831-1879) who explained the fundamental nature of light as electromagnetic waves (oscillating electric and magnetic fields; EM waves) through his four equations. Maxwell equations not only describe light waves that we perceive with our eyes, but also tell us that light is a tiny part of a whole spectrum of waves extending from nuclear gamma rays to radio waves. The EM wave spectrum is explained in the following slides.
(Click on a slide to see its full page image)
(You can skip the slides without loss of continuity)
Okay, so Maxwell accurately described all observations like reflection, refraction, difraction, interference, polarisation, dispersion etc. of light waves, but what about observations, such as the atomic spectral lines or the photoelectric effect, which just could not be understood if we consider light as waves (Maxwell's equations). Einstein (1879-1955) showed that to understand these, one has to consider that light is made up of a stream of particles (photons). But photons, as particles, could not bend round corners (diffraction) - only waves can do that. This seems all very confusing. Is light a wave or a stream of particles?
We need the complex theories of Schrodinger and Heisenberg to make sense of the situation - suffice to say that such confusing behaviour appears to be a fundamental feature of nature applicable to all matter.
How is Light Generated? - There are two main categories of light.
(a) Light that has a wide range of wavelength distribution - a good example is white light from the Sun or from a light bulb. Bodies at about 5000oC are the most efficient in radiating energy in the visible region. Such radiation is called the black body radiation and contains a large range of wavelengths - in the case of the solar radiation the wavelength spread (full width at half maximum - FWHM) is ~700 nm.
The second type of light has an extremely narrow FWHM (for atoms it is typically of the order of 0.01 nm) - it essentially appears as a line on the spectral graph - the width of the line generally represents the resolution of the spectrometer. Such light is emitted by excited atoms and molecules - we can excite atoms in solids by heating the sample; in gases an electric discharge is very effective. An example of the line spectrum of hydrogen is shown below:
If white light is passed through an element in gaseous form, then it absorbs the same wavelengths that it would emit in its emission spectrum - this results in a white light spectrum with narrow dark lines (absorption spectrum).
Elements and molecules have unique line spectra. Such line spectra are very useful in elemental analysis of samples - we use absorption spctra to determine what elements are present in stars and space in general (see slide above for the Sun), in forensic work etc.
A fascinating example of how bodies radiate and absorb light energy is discussed, with detailed examples, in my publication on the science of climate change.
Lasers - A laser is an unusual light source - it produces a beam of light which has an extremely narrow spread of wavelengths (can be much lower than 0.0000001 nm).
Laser beams do not spread out much as they travel large distances - a laser used in measuring Earth-Moon distance (384,400 km) directs a laser beam to the Moon, and the spot at the Moon only grows to 10 km in size. The time it takes for the laser light to return back to Earth allows the Earth-Moon distance to be measured to millimeter precision!
It has been determined that the Moon is drifting away from the earth at a rate of 3.8 cm per year.
A laser beam may be focused to very small size and the energy density in the laser spot can be billion of times larger than the solar light intensity on Earth. The ability to deliver such large power to small areas makes lasers very useful in industrial context.
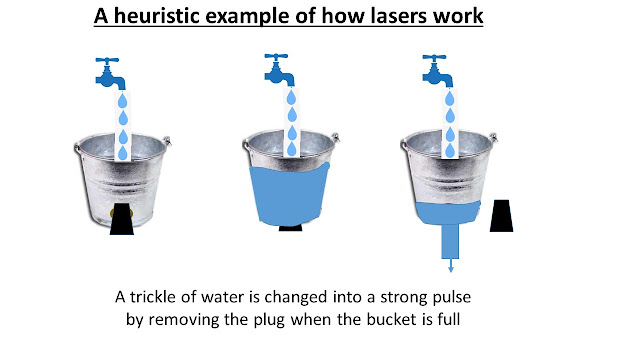
In fact lasers play essential roles in many aspects of our lives. Without going in detail, I shall list some applications of lasers; a detailed account of laser applications may be found by clicking here. Some of the applications are also described in a very readable form here. How Do Lasers Work? - I shall use a heuristic approach for this section. (See the slide below) Let us consider a water bucket with a big hole at the base - the hole has a plug to avoid leaks.
Water trickles into the bucket and fills it up - let
us say in one hour. When the bucket is full, the plug at the base is
removed and the bucket-full of water gushes out in a couple of seconds.
What we have done is changed the trickle of water into a strong pulse of flowing
water.
Lasers work on a similar principle. In order to keep it simple, I shall
describe a 3-level laser system in a non-rigorous way. Normally, all
atoms in a material are in their lowest energy state - the ground state.
By supplying external energy (for example from an arc lamp), some of the atoms
can be moved to excited states. Atoms in the excited states shed their
extra energy by emitting a photon after a short period of time - typically of
the order of a few nanosecnds (a billionth of a second). If we choose our
material carefully, then the atoms might also have a long-lived excited state
(a meta-stable state) with a lifetime of a few milliseconds or longer.
An atom in a short-lived excited state
could decay to this meta-stable state. After a few microseconds, there
will be a lot of atoms in this state (see slide below).
When an atom in the meta-stable state
decays, it will emit a photon with an energy equal to the excitation energy of
the meta-stable state. According to Einstein, this photon (or the wave packet)
will stimulate, almost instantaneously, the de-excitation of all the other
atoms in the excited meta-stable state. The result will be a
pulse of light in which all the wave packets are in phase and are travelling in
the same direction. Essentially, the energy that the arc lamp had produced has been
concentrated into an orderly, almost non-diverging, light pulse of a very short
duration. This is a laser.
Thanks for reading. Please pass
the link to your friends and family. Comments are welcomed.













No comments:
Post a Comment