Friday, 12 August 2011
You have enough DNA to circle the Earth 4 million times!!
Unbelievable - each of us has enough DNA to circle the Earth 4 million times.
This is how it works:
If stretched, DNA in a single cell will extend to 1.5 metres**
A cell is typically 10 microns (1 micron = 10-6 m) across
Volume of a cell is of the order of 10-15 m3
Consider a 100 kg man with density equal to that of water (1000 kg/m3)
Volume of the person is 0.1 m3 (volume = mass/density)
Therefore, there are 1014 cells - this is 100,000 billion cells and
the length of the DNA is 150 billion km
This is sufficient to circle the Earth 4 million times!
** Human Genome Project has mapped 3 billion base-pairs in a human DNA. If base-pairs are 0.5 nm apart then the stretched DNA would be 1.5 m long.
Earth's circumference is about 40,000 km
Sunday, 19 December 2010
Brownian Motion - Atoms are Real afterall!
Even at the end of the 19th Century, scientists couldn't decide if atoms were real or just a convenient way to be able to calculate bulk properties of matter. Good amount of debate was going on - to the chemists it looked that atoms are real but the science of thermodynamics did not need atoms and thermodynamics was the queen of sciences at the end of the 19th Century with the Industrial Revolution providing the means of creating wealth and creating the feel-good factor in plenty.
How Einstein demonstrated the reality of atoms and counted them is fascinating.
I have published the slides of my talk on Einstein and the Theory of Relativity in the following to demonstrate how Einstein worked it out... (Click on the image for a full screen view)

Saturday, 18 December 2010
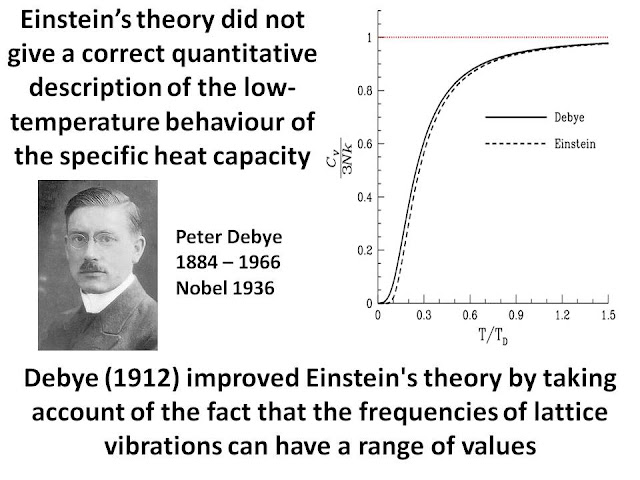
Specific Heat of Solids... Another problem with Classical Physics!
Towards the end of the 19th Century, classical physics had serious difficulties in explaing many observations. Einstein provided a solution of the specific heat of metals problem by assuming that energy absorption is quantised and is not continuous. This was a radical departure from the way physics was done in the 19th Century and demonstrates yet again the bold and visionary nature of Einstein's genius.
In the following I have published my slides from the talks on Einstein and the Theory of Relativity...
In the following I have published my slides from the talks on Einstein and the Theory of Relativity...

Sunday, 12 December 2010
Einstein Rides a Light Wave...

This is a thought experiment Einstein might have done (not necessarily in the way I have described but the idea is interesting)
A light wave is an electromagnetic wave (EM Wave) which has oscillating electric and magnetic fields. Suppose Einstein travels with the wave at the speed of light! What will he see?
A light wave is an electromagnetic wave (EM Wave) which has oscillating electric and magnetic fields. Suppose Einstein travels with the wave at the speed of light! What will he see?
He will see a constant electric field - this will not generate any oscillating magnetic field which in turn cannot generate an electric field. There will be no oscillating electric and magnetic fields that typify an EM wave. It would seem that no wave would appear to exist.
This is the interesting part - if you travel with the speed of light then you can not transmit or receive information: there are no EM waves.This encouraged Einstein to postulate that speed of light is the maximum speed allowed and nothing can move with a speed greater than the speed of light.
This is the interesting part - if you travel with the speed of light then you can not transmit or receive information: there are no EM waves.This encouraged Einstein to postulate that speed of light is the maximum speed allowed and nothing can move with a speed greater than the speed of light.
Not tremendously convincing but the idea is interesting.
Friday, 10 December 2010
On the Nature of Light: Wave-Particle Duality
Three fundamental quantities that help us to perceive the world around us
Space 3-D space - We can use a ruler to measure this
Time - Use a clock - atomic clocks can be accurate to 1 sec in a billion years
Means of transmitting information – LIGHT or EM waves in general…..
The speed of light is the fastest speed at which information/energy can travel
Speed of light is 300,000 km per second
The finite speed of light means that the time we receive the signal is later than the time the signal left the source (we are observing what happened in the past). This situation may be different for different observers and can create some bizarre effects.
Light plays a fundamental role in the theory of relativity.
Puzzling behaviour of light: Sometimes light acts as a wave while at other times it behaves like a stream of particles (photons) - Light displays a dual nature
But first a brief history:
1675 - Isaac Newton thought that light was composed of corpuscles (particles of matter) which were emitted in all directions from a source. Newton Published Optiks in 1704
Despite some serious problems with this view, Newton’s reputation helped the particle theory of light to hold sway during the 18th century
Robert Hooke (1635 - 1703) proposed in 1660 a wave theory of light.
Christiaan Huygens (1629 - 1695)in 1690 suggested that light was emitted in all directions as a series of waves in a medium called the Luminiferous ether
Thomas Young (1773 – 1829) in 1801 performed the Double-slit Interference Experiment.
Some say that it is the most beautiful experiment in physics.
The observations of the double-slit experiment could only be explained if light behaved as waves...
In 1860s, Maxwell combined the fields of electricity, magnetism and light and predicted the existence of electromagnetic (EM) waves. In his brilliant and very successful theory, Maxwell showed that all EM waves travel at 300,000 km/s which was the same as the measured speed of light.
Light is the “visible” EM wave
EM waves span a wide spectrum from radio waves to nuclear gamma rays
It was thought that as a wave motion, light would require a medium to travel - such a medium was called ether which was supposed to pervade the whole universe and had some really bizarre properties like being extremely stiff to allow vibrations of very high frequencies, be extremely thin (rare) to allow unimpeded motion of planets and objetcs through it etc. Attempts were made to measure the speed of the Earth relative to the ether but even the most sensitive experiments failed to find any evidence of the expected relative motion.
This caused a big headache for physicists at the end of the 19th Century.
Thursday, 9 December 2010
Einstein suggestes quantum nature of Light - Photoelectric Effect
Photoelectric Effect - Another Problem for Classical Physics
Photoelectric effect is the emission of electrons from a metal surface by gaining energy from light.
First observed by Heinrich Hertz (1857 – 94) in 1887
Studied in detail by Philipp Lenard(1862 – 1947) in 1902
Lenard was awarded Nobel Prize in 1905
According to classical physics, the metal surface simply soaked up light energy and sooner or later, depending on the intensity of the light, it would accumulate enough energy to cause emission of electrons.
Experiments did not verify this and cast doubt on the validity of the wave theory of light – a cornerstone of Maxwell’s theory of electromagnetic waves
Experimental results were:
1. If the light is below a certain frequency f0 , then no electrons would be produced however long we shine it on the metal surface.
2. For light frequency above f0 electrons would be produced immediately, with no time delay even for extremely weak light intensities
In 1900, to explain a problem with black-body radiation spectrum Planck had made an ad hoc assumption that emission and absorption of energy can occur only in discrete amounts.
Einstein made the bold assumption that light consists of a stream of particles; the energy of individual particles or quanta is determined by the frequency of light. Einstein’s light quanta are now called photons
Energy of a photon is E = h x f where h is the Planck’s constant.
According to Einstein, in photoelectric emission, a light photon penetrates the metal and knocks an electron loose. A minimum energy is required to knock off an electron from the metal. Hence photons below a certain frequency f0 do not have sufficient energy (= hf0) to release electrons. Increasing the intensity of the light increases the number of photons and hence the number of electrons emitted would increase.
Einstein’s theory raises a fundamental question:
Is light a wave, or a stream of photons?
Studied in detail by Philipp Lenard(1862 – 1947) in 1902
Lenard was awarded Nobel Prize in 1905
According to classical physics, the metal surface simply soaked up light energy and sooner or later, depending on the intensity of the light, it would accumulate enough energy to cause emission of electrons.
Experiments did not verify this and cast doubt on the validity of the wave theory of light – a cornerstone of Maxwell’s theory of electromagnetic waves
Experimental results were:
1. If the light is below a certain frequency f0 , then no electrons would be produced however long we shine it on the metal surface.
2. For light frequency above f0 electrons would be produced immediately, with no time delay even for extremely weak light intensities
In 1900, to explain a problem with black-body radiation spectrum Planck had made an ad hoc assumption that emission and absorption of energy can occur only in discrete amounts.
Einstein made the bold assumption that light consists of a stream of particles; the energy of individual particles or quanta is determined by the frequency of light. Einstein’s light quanta are now called photons
Energy of a photon is E = h x f where h is the Planck’s constant.
According to Einstein, in photoelectric emission, a light photon penetrates the metal and knocks an electron loose. A minimum energy is required to knock off an electron from the metal. Hence photons below a certain frequency f0 do not have sufficient energy (= hf0) to release electrons. Increasing the intensity of the light increases the number of photons and hence the number of electrons emitted would increase.
Einstein’s theory raises a fundamental question:
Is light a wave, or a stream of photons?
Wednesday, 8 December 2010
Black Body Radiation - Planck's Solution
(Planck’s assumption of) ...the atomistic (quantum) structure to energy, governed by the universal constant h ... became the basis of all twentieth-century research in physics and has almost entirely conditioned its development ever since. ... Albert Einstein 1950
Every body radiates energy over a range of wavelengths.
How much energy is emitted at each wavelength (shape of the curves) is predicted by the classical theory. Well established theories of Thermodynamics, Statistical mechanics and Electromagnetism
form the basis of such predictions.
In 1900 Planck suggested that physics should abandon the assumption that energy is continuous and wavelike. Instead, if energy can only be absorbed and emitted in discrete packets (or quanta now called photons ), theory can be made to fit observations exactly.
Energy of a quantum = h x its frequency
Planck was awarded Nobel Prize for his work in 1918. Planck and Einstein became good friends. Planck supported Einstein during the turbulent period when Jews were being prosecuted in Germany.
Every body radiates energy over a range of wavelengths.
How much energy is emitted at each wavelength (shape of the curves) is predicted by the classical theory. Well established theories of Thermodynamics, Statistical mechanics and Electromagnetism
form the basis of such predictions.
In 1900 Planck suggested that physics should abandon the assumption that energy is continuous and wavelike. Instead, if energy can only be absorbed and emitted in discrete packets (or quanta now called photons ), theory can be made to fit observations exactly.
Energy of a quantum = h x its frequency
Planck’s suggestion was ad hoc and had no theoretical basis This troubled a lot of people including Planck. Planck believed in the wave nature of light (energy) and insisted that it might be in the way individual matter atoms interact with radiation that gives rise to the quantum aspects in the emission of radiation. It was Einstien who in 1905 first suggested that light (energy) is actually quantised following Planck's equation.
Planck was awarded Nobel Prize for his work in 1918. Planck and Einstein became good friends. Planck supported Einstein during the turbulent period when Jews were being prosecuted in Germany.
Subscribe to:
Posts (Atom)