Blog Contents and Who am I?
Water is an amazing substance. Water is everywhere. Water is a
compound made from hydrogen and oxygen - two elements that are the most
abundant in the solar system (besides helium).
Ø Water covers 70% of the Earth's surface.
Human body is 60+% water, as are most animals. Plants can be up to 95%
water. The biosphere seriously depends on water for its function
and survival.
Ø Water makes photosynthesis possible and enables
production of food on which all living creatures depend for energy.
Ø Water is vital for supporting living organisms - in
thermal regulation, transport of oxygen and nutrients, biochemical reactions,
stability of cellular structures; many functions of the body depend on
the availability of water and no other substance can replace it.
Ø Gases in the atmosphere act as a blanket that
keeps the Earth about 33⁰C warmer than it would be otherwise - making
survival of complex life possible. Water vapour provides about 60% of
this 'insulation' and prevents heat energy escaping from the earth to
outer space.
Ø Water in the oceans absorbs 94% of incident solar
energy and helps to distribute this energy over the rest of the
Earth. At normal environmental temperatures, water is found in all three
states (solid, liquid & gas).
Ø Without water, it would be impossible
to maintain a steady planetary temperature and avoid severe short term fluctuations. Earth's
climate critically depends in the way water is distributed geographically.
Ø Water is called the 'universal solvent' - in rivers
it brings nutrients essential for agriculture. Water helps to dispose
human waste, is vital for practically all industrial/manufacturing activity
- complex civilizations critically depend on the availability of water -
droughts (lack of water resources) have been responsible for the fall of many
historic civilizations.
The question is - what makes water so
important for life and why no other substance can replace it?
Compared with other materials, water has
some extreme and unusual properties. Without these, earth's climate would be
seriously hostile, and the life as we know will not be able to function.
Slide 1:
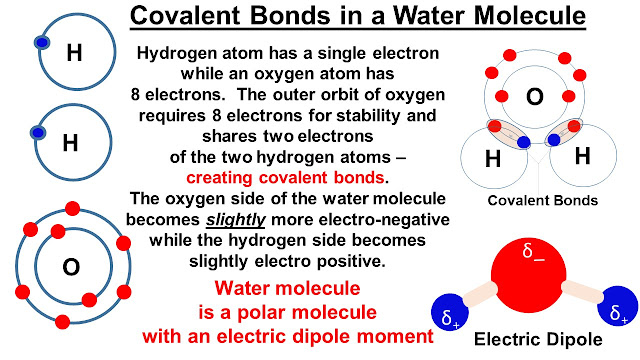
The chemistry of water, H₂O, is fascinating and mostly revolves around the way a water molecule (itself formed by the combination of one oxygen and two hydrogen atoms - covalent bonding - see slide 2) can attract other water molecules (cohesion) and also attract many other chemicals (adhesion) by forming hydrogen bonds (H-bonds). The science of the formation of H-bonds is discussed in more detail in the Appendix. It is also worth pointing out that in water the covalent bond (intramolecular force binding oxygen and hydrogen molecules) is 20 times stronger than the hydrogen bond (intermolecular foce binding two different water molecules) - the covalent bond has a strength of ~ 460 kJ/mole while H-bonds are 23 kJ/mole.
Slide 2:
Many physical properties of water molecules (properties 1 to 8 in slide 1) are determined by inter-molecular forces like the hydrogen bonds.
In the following, I describe how unique and extreme many properties of water are in comparison to other substances.
Liquid Water is Heavier than Ice - In the solid state, all substances have a higher density (mass per unit volume) than in the liquid state - except water. Water has the unique property that liquid water is heavier than it is in solid phase (ice). The freezing point of water is 0⁰C.
Slide 3:
Kinetic energy (energy of motion) of molecules increases linearly with temperature - at higher temperatures molecules move about more vigorously. In the case of water in solid state (ice), molecules are held in place in an hexagonal (six-sided polygon) crystalline structure with lot of empty space (for a 2-D view, see slide 4). In ice, the slightly electropositive hydrogen atoms of a water molecule form hydrogen bonds with the electronegative side of oxygen atoms of two other water molecules (see slide 4a and Appendix for hydrogen bonding). In liquid water, due to thermal motion hydrogen bonds are constantly broken and re-formed hundreds of billions times per second - allowing some water molecules to have on average somewhat smaller spacing (higher density) than in the rigid ice structure.
Slide 4: 
Slide 4a:
The strength and number of H-bonds that each water molecule can form give water its unique density profile. As temperature is raised above zero degree, only a very small number of H-bonds are broken; thermal motion is not sufficiently energetic to increase average spacing between water molecules (at 0⁰C, there are still 3.69 H-bonds per water molecule present), but free water molecules can enter the empty spaces of the ice crystal - the result is that the voids in ice are reduced, and the density of water increases as ice melts. It is only above 4⁰C that the increased spacing due to thermal motion causes the density of water to start decreasing with rising temperature.
A well-known consequence of water being heavier than ice is that in colder parts of the earth (higher northern and southern latitudes), during winter months when the ambient temperature falls below freezing, the top surface of water in lakes freezes. Ice being lighter than water stays on top - floats above the liquid water below. Ice is also a very poor conductor of heat and the heat loss from water below it is greatly reduced with the result that water stays liquid for much longer. As ice thickness increases, the water under it is insulated even more effectively. The liquid water enables aquatic life in the lakes to survive.
We all know that pot holes appear in roads during the winter season. This happens because small cracks in the road surface are filled with water. This water freezes when the temperature falls below freezing. As ice has a larger volume than water, the newly formed ice creates a strong stress on the surrounding tarmac and cracks it - this eventually grows in size and damages the road surface.
On a larger scale, a similar process is responsible for disintegrating rocks (mechanical weathering of rocks) when water freezes in the cracks making them bigger. In the next cycle, water would have seeped deeper into the now-bigger cracks. The process continues until the rock disintegrates.
Food from the freezer does not taste as good as fresh food. The reason is that in frozen food, water in the cells freezes, expands and damages some of the cell membranes. The liquid from the cells changes the texture and taste of the food. If you refreeze the food then even more cell walls will be damaged resulting in further loss of quality. Refreezing ice-cream is a particularly bad idea.
High Melting and Boiling Points: Water has abnormally high melting and boiling points compared with other similar compounds (slides 5 and 6). This is due to the strength and number of hydrogen bonds (H-bonds). A water molecule can form H-bond with four other water molecules (see slide 4a). Due to the presence of large number of relatively strong H-bonds, one requires considerably increased thermal motion to make individual water molecules free - it is estimated that even at 100⁰C, on average a water molecule has 3.24 H-bondings with neighbouring molecules.
Slide 5:
The above slides show the systematics that within a group, melting and boiling points decrease when hydrogen covalently combines with elements of a lower period - see slide A1 in the Appendix for details of the periods and groups of the periodic table; without H-bonding, water would freeze around -110⁰C and boil at around -75⁰C. If water was like other hydrides then most water on earth will readily evaporate and eventually leave the earth's atmosphere (we need to thank H-bonds that we have water on earth!).
Water is the only substance that is present in solid (snow and ice), liquid and gaseous (water vapour, clouds) forms at temperatures that naturally occur on the earth. Our planet's weather pattern is driven by the ability of water to vaporise at prevailing temperatures - solar energy is mostly received in the tropics but is distributed over the rest of the globe by the heat carried by atmospheric water vapour and by ocean currents. Without regular rainfall, many parts of the world will not be able to sustain agriculture and support large populations.
High Specific Heat Capacity, Latent Heats of Fusion and Evaporation:
Slides 7, 8 and 9 show that water has exceptionally high values for the above physical properties.
Slide 7:
Slide 9:
 Again, these properties of water owe their extremely high values to the number and strength of H-bonds present. The above three slides compare water with other common substances found in nature and it is obvious that water has extreme values. Let us recap what information is there:
Again, these properties of water owe their extremely high values to the number and strength of H-bonds present. The above three slides compare water with other common substances found in nature and it is obvious that water has extreme values. Let us recap what information is there:Specific Heat Capacity (C): This is the amount of energy required to increase the temperature of a unit mass of liquid water by 1⁰C. For water it is 4.18 Joule per gram or 1 calorie per gram.
Latent Heat of Fusion (Lf): Amount of energy required to melt a unit mass of ice at 0⁰C. It is 334 Joule per gram or 80 calorie per gram.
Latent Heat of Vaporisation (Lv): Amount of energy required to vaporise a unit mass of water at 100⁰C and 1 atmospheric pressure. It is 2265 Joule per gram or 541.3 calorie per gram.
Lf and Lv have such high values compared to C because in order to heat liquid water, we are simply breaking some H-bonds to let water molecules move about a bit more freely. For fusion, we are disturbing the rather stable crystal structure of ice by removing water molecules and on average more H-bonds must be broken to do so. Lv has the extraordinarily high value, 541 times bigger than C, and represents the need to break all H-bonds and supply enough thermal motion to water molecules to break them free from the water surface (water surface tension is also abnormally high - this is discussed later).
Temperature Regulation: Slide 7 tells us that it takes seven times more energy to heat water than heating sand, concrete or brick. In the environment, light brings energy from the Sun and is absorbed by the earth's surface. Most surfaces on land become quite hot but water in the lakes and ocean never gets very warm. Warm air flows from land to water bodies during the day and helps to cool land surfaces. The wind direction reverses during the night when land surfaces have cooled rapidly.
Water covers 70% of the earth's surface and with its large C value, acts as a heat reservoir without letting the ambient temperatures rise/fluctuate too much. The large value of the specific heat of water helps to minimize temperature fluctuations helping life to survive.
Human body maintains a core temperature of 37⁰C and will stop functioning at 42+⁰C. When exercising or in high ambient temperatures or if you have high fever, the body produces sweat (sweat is mostly water). Water in sweat evaporates to cool the body. The energy required to evaporate water is drawn from the body and as Lv has a large value, sweating is a very efficient way for regulating body temperature.
_____________________________________________________
Have you read?
-----------------------------------------------------------------------------
Surface Tension and Capillarity of Water: Surface Tension is the tendency of liquid surfaces to shrink into the minimum surface area possible.
Slide 10:
Water has the highest surface tension of all liquids - except mercury. Mercury is a metal, and metallic bonding forces are extremely strong; this makes the surface tension in mercury very large - about 6 times greater than water. Slide 11 lists surface tension values of common liquids:
Slide 11:
Air bubbles inside water are spherical because the trapped air is surrounded by water. Water surface tension forces tend to minimise the surface area of the bubble causing it to shrink in size resulting in an increase of air pressure inside the bubble. The bubble stabilises when the inward pressure due to surface tension force is equal to the ourward air pressure inside the bubble. Slide 12 shows an example of air bubbles inside a glass of cold tap water left undisturbed for 24 hours (air bubbles form in standing water as air is less soluble in warmer water). Capillarity: Capillary action allows water to rise against the force of gravity inside narrow spaces. Capillary action is due to cohesive and adhesive forces. Cohesive forces are attractive forces between molecules of water and give rise to surface tension effects (see slide 10). Adhesive forces are between molecules of liquid water and those in the walls of the solid container (generally glass or plastic). Adhesive forces can be greater than cohesive forces and result in the water being pulled up in the container even against the force of gravity (capillarity or wicking). The rise is inversely proportional to the radius of the tube (in a glass tube of radius 1 mm, water rises by 14 mm).
There are many examples (1, 2, 3) of capillarity and surface tension at work in nature, biology, medicine and industrial processes.
Water as a 'Universal' Solvent: Water is an amazing solvent - water dissolves more substances (but not all) than any other liquid. Water is ubiquitous, and always on the move in the environment and in living systems. Water transports and delivers valuable chemicals, minerals, nutrients to make life possible. What makes water such a good solvent?
Water molecule is a polar molecule with a large electric dipole moment (see slide 2). Remember that while a polar molecule is overall uncharged, the internal distribution of charges is such that there are partially negative (the oxygen side in water) and partially positive regions (the hydrogen sides). The overall effect is that the molecule has a non-zero dipole moment. The dipole moment of water is 1.84 Debye (1 Debye = 3.33 x 10⁻³⁰ Coulomb.meter) and is larger than most other polar liquids.
Thus, water molecules feel strong attraction to other polar molecules, so much so that it can disrupt the attractive forces that hold the individual atoms of the molecule together and dissolve them. For more details please click here.
Fats, oils and most hydrocarbons do not have regions of partial positive and negative charges (they are non-polar molecules). Electric attraction forces between water and oil & fat molecules are weak. Therefore, they do not dissolve in water.
Slide 13:
It is worth mentioning that nonpolar solvents (shown red in slide 13) are excellent solvents for dissolving other nonpolar solutes (for example, hydrocarbons like wax). A general rule is that 'like dissolves like'.
Final Word: Water in the atmosphere not only provides the necessary blanket to maintain Earth at a habitable temperature, water in the oceans absorbs most of the Sun's energy falling on the Earth's surface and distributes it around the globe. Water in the oceans also dissolves atmospheric CO₂ to reduce its amount in the atmosphere. Dissolved oxygen in water makes aquatic life possible. I shall discuss the extremely important role of atmospheric water and oceans in the next article but leave a summary of how atmospheric water vapour does an amazing job of keeping us all warm.
Slide 14:
APPENDIX: I discuss the science of formation, and properties of hydrogen bonds in the following. H-bonds are intermolecular attractive forces that connect two molecules. They are formed between a hydrogen atom of one molecule and a lone electron pair of a strongly electronegative atom (nitrogen, oxygen or fluorine - the three most electronegative elements) of another molecule.
Note: H-bonds are much stronger than the relatively weak attractive van der Waal bonds which operate between neutral molecules. Also, H-bonds should not be confused with much stronger intramolecular bonds (covalent, ionic & metallic bonds) which operate within a molecule.
Slide A1:
Slide A2:
H-bonds are formed between functional groups F-H, O-H or N-H of two molecules and are represented by 3 dots or dashes between H of one molecule and N, O or F of a neighbouring molecule. H, N, O and F have the smallest sizes of all atoms (slide A2) and with the greatest difference in electronegativity between H and N,O,F atoms (slide A1). Functional groups F-H, O-H and N-H have highly concentrated partial charges (see slide 2 in the main text) and can form relatively strong electrical dipoles. The individual molecular dipoles strongly attract to form the so-called hydrogen bonds between molecules. The obvious examples are the three hydrides NH₃, H₂O and HF (see slide A3) of which H₂O has the most extensive hydrogen bonding - one molecule of water can H-bond with four other water molecules, giving water many unique and extreme properties (without these life on earth will not be possible!). Slides A3 and A4 explain the situation, also see slide 4a in the main text:
Slide A3:
Hydrogen bonds play a vital role in determining many of the physical and chemical properties of water. H-bonds are also fundamental to the stability of DNA double helix structure, folding and managing 3-D shapes of proteins and formation of many organic polymers like cellulose etc.
The fact that a water molecule can form four relatively strong H-bonds with its neighbours gives liquid water strong cohesive (sticking to other water molecules) and adhesive (sticking to molecules of other substances) properties. To make a water molecule free, one needs to break several H-bonds and this requires input of lot of energy. In the main text, I discuss how water is unique in having extremely large latent heats of fusion and vaporisation, very high melting and boiling points, abnormally large specific heat capacity, surface tension and an anomalous density-temperature profile. These extreme properties are due to a water molecule's ability to form H-bonds with other water molecules.
Adhesion also plays a fundamental role in nature - capillarity allows a plant to transport water to all its branches and leaves.
H-Bonds in Other Chemical Compounds: Hydrogen bonds are present in a large number of compounds. In the following slides, I mention a few such bond formations that are relevant to biological molecules, but refer you to the general literature for details.
DNA: The double helix structure owes its stability to hydrogen bonds between two set of base pairs A---T and C---G. AT and CG are the only bonds found in DNA structure. AT H-bonds are H---O and N---H while CG bonds are formed as H---O, N---H and O---H.
Slide A6:
Cellulose: is the most abundant organic polymer, making up to 90% of cotton and 50% of wood, is important constituent of plant cell walls and extensively used to make paper, textiles and other industrial materials. Cellulose is a straight chain polymer consisting of several hundred to many thousands of linked (C₆H₁₂O₅) units (is a polysaccharide - many sugars) The C₆H₁₂O₅ structure has multiple hydroxyl groups (-OH groups). Hydrogen atoms of the hydroxyl groups from one chain form hydrogen bonds with oxygen atoms on the same or on a neighbouring chain, holding the chains firmly side-by-side (see slide A7), forming microfibrils (thin fibre-like strands). Because of H-bonding, microfibrils are extremely strong, tough and inflexible, and provide cellulose high structural strength.
Slide A7:
Proteins: Proteins are integral part of the body, and are responsible for the support and repair of body tissue. All enzymes are proteins, as are antibodies, haemoglobin in blood, myacin in muscles etc. All proteins are made up from 20 amino acids which are joined together by covalent peptide bonds (slide A8). In a prptide bond, carbonyl group (C=O) of one amino acid is covelently bonded to the amino (N-H) group of another amino acid. Proteins are polypeptides and may contain hundreds of peptide bonds.
The -C=O and -N-H groups are polar and can form H-bonds between diffrent peptide bonds. Hydrogen bonds are responsible for secondary structrures of proteins and for their functions. Slide A8 (adapted from Wiki) shows an example of H-bond formation between two polypeptide chains.
Slide A8:
The above examples demostrate that H-bonds play a primary role in the functioning of most biological systems.
Copyright: Some figures are modified Wiki figures but most figures have been drawn by myself and may be used provided the blog address is acknowledged.





















No comments:
Post a Comment