The fundamental question in Geology has been - what is the age of the Earth? A significant step in this direction was the realization by James Hutton followed by Charles Lyell and others in the 18th and 19th centuries that rocks recycle over time - they form, erode, sediments collect and compress to form new rocks - the process repeating again and again. The sediments include in them remains of plants and animals that inhabit the Earth at that time - fossils - and the layers of such sedimentary rocks are essentially a catalogue of geological events in a time ordered fashion - just the absolute time scale is missing. Grand Canyon is an excellent example.
The following few slides summarize the position:
(please click on a slide to view its bigger image)
The way I think of the relative geological time scale is like a stack of books arranged in the correct chronological order of the historic events as they happened but with no dates provided - as I show in the next slide
It is not that people did not try to determine a value for the age of the Earth. It is just that the understanding of the physical processes was not good enough and all attempts gave results which were wide off the mark. Even the church got involved and adopted the ridiculously small value of 6000 years as dogma and caused a lot of grief to lot of people. The next few slides explain some of the attempts made



I must say that physicists and astronomers also did not help in the process of finding the correct age of the Earth. Lord Kelvin did not know about convection currents in the molten magma in the interior of the Earth nor did he know about radioactive elements which were a source of heat energy. But Kelvin was an eminent scientist and everybody listened carefully to what he said - in 1900 he said that the Earth is 20 million years old and that was something you do not challenge. Evolutionary biologists did object to such a young Earth as this did not allow enough time for species to evolve etc. - a great debate - often ill tempered - ensued but there was no way out of the dilemma as to how to find a true reliable value of the age of the Earth.
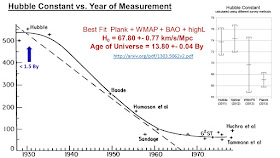
In the 1920s and 30s, radio-isotope dating was getting established and was measuring ages of some rocks as 2 or more billion years. Astronomers were discovering that the Universe is expanding and theorizing that the Universe might have started from a small compact volume less than 1.5 billion years ago. The Earth has to be younger than the Universe and this gave geologists a great deal of ammunition to aim at scientists like Arthur Holmes who believed that radio-isotope dating was a method that is capable of providing an absolute age of the earth. It should be stated that the astronomers have got their act together and in 2015 measured the Hubble constant and hence the age of the Universe as 13.8 Billion years with a tiny uncertainty.
Radioactivity was discovered in 1896 and soon it was proving useful in medical applications. Rutherford was the first scientist to apply it for determination of age of geological samples. Systemic errors were serious and the science of radioactivity was not understood well at that time. Even isotopes were not known until about 1915.
This certainly gave the established geologists a big excuse to criticize the new science and it was really the solo fight by Arthur Holmes that eventually brought radio-isotope dating technique to universal acceptance by about 1950.
Let us first look at the radioactive isotopes of interest for geological dating. It is believed that the Solar System was formed from a distribution of matter known as the Solar Nebula. As the nebula contracted, most of the matter collapsed to form the Sun and a tiny percentage of the nebula formed the planets, asteroids, meteorites and the comets. All of this happened over a very short period of a few hundred million years at most and it would be correct to say that our Earth formed at the same time as the Sun and the meteorites. In fact, we can plot the abundance of elements found in the Sun and compare this to elemental abundance of early formed meteorites and find an exact correlation in these abundances. This is shown in the slide
It is now accepted that meteorites are the best objects for dating the solar system. They were formed early on in the process for the nebula contracting and the chondrules in the meteorites have preserved their purity and initial composition at the time of their formation. Claire Patterson have actually shown that the chondrules have similar age as some of the ocean sediment rocks confirming that they were formed at roughly the same time.
The next question is which radio-isotopes are useful for determining the age of the earth. Unstable isotopes decay with a characteristic rate - called the half-life. Half life is the time that an isotope will require for half of the original amount to decay to another isotope (called the daughter). The daughter isotope may or may not be stable and can decay in turn but eventually produces a stable isotope that maintains its quantity over time. The best isotopes are those whose half lives are similar to the age of the earth - a few billion years.
The most frequently used isotopes for dating meteorites and earth's crustal rocks are those of uranium-238 and rubidium-87.
For dating oceanic crust, K-40 is a good choice. These are shown in the next slide
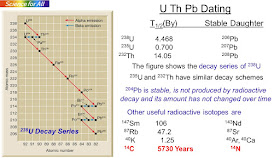
Carbon -14 has a short half life of 5730 years and is only useful for dating samples that are less than about 50000 years old.
Modern mass spectrometers are very sensitive and provide sufficient sensitivity to measure the tiny amount of radioactive isotope or the daughter product that is present in the sample.
Meteorites are good approximation to a closed system - particularly the small spherical chondrules, that meteorites contain, are very stable and have undergone little or no change since they were formed 4.65 billion years ago.
Zircon is an ideal mineral for dating terrestrial rocks.
In an actual analysis to determine the age of a sample, it is necessary to measure the abundance of the parent, daughter isotopes and also we need to know how much of these isotopes were present at the time of formation. How these abundances are related to the elapsed time (age of the sample) is determined by radiometric equations which are described in this article. Essentially, an isochron is constructed - isochron is a straight line graph whose slope gives the age of the sample.
The first comprehensive measurement of the age of the earth was done by Claire Patterson in 1956 and we present the results obtained by Patterson which still stand. By the way, Patterson was responsible for banning of leaded petrol - this he achieved through a thirty year struggle with the government bodies and the petrochemical industry!
In conclusion, it seems reasonable to say that the Earth came in existence at the same time as the Solar System which is about 4.568 billions of years old.
Blog Contents - Who am I?
Please send your comments to ektalks@yahoo.co.uk



























No comments:
Post a Comment